Glaucoma & Optic Neuropathies
Accelerating Glaucoma & Optic Neuropathies clinical trials through efficient operational models, innovative endpoints and streamlined global execution.
Unsurpassed expertise in Glaucoma & Optic Neuropathies led by one of world’s leading KOLs in the field.
Glaucoma & Optic Neuropathies are one of the leading causes of irreversible vision loss worldwide. The number of patients suffering from these debilitating diseases is expected to exceed 110 million by 2040. For Glaucoma, traditional IOP reduction treatments simply aren’t enough, as patients continue to experience optic nerve damage. For other Optic Neuropathies, few options are available.
With 30+ years experience in Glaucoma research, Ora supports next-generation therapies by combining global expertise, advanced endpoints, and a sponsor-first mindset to speed approval. Ora has led 20+ programs in the past five years, driven by a deep understanding of the disease’s clinical nuances. Our expert team will take your novel therapy to the next level, and mitigate risk at every stage.
Work with Ora, the ophthalmology research company and give your state-of-the-art Glaucoma & Optic Neuropathies therapeutic or device the competitive edge it needs to win the race to be first to market.
Key Indications
Primary Open Angle Glaucoma
Intraocular Pressure
Ocular Hypertension
Neuroprotection
The Ora Glaucoma & Optic Neuropathies team leaders
Ora Glaucoma & Optic Neuropathies experience over the last 5 Years
Patients enrolled
Sites activated
Programs
The Ora Glaucoma & Optic Neuropathies team’s comprehensive expertise spans early-phase clinical trials to late-stage studies. Our strategic regulatory guidance and efficient operational execution, ensures seamless trial progression through even the most complex development pathways.
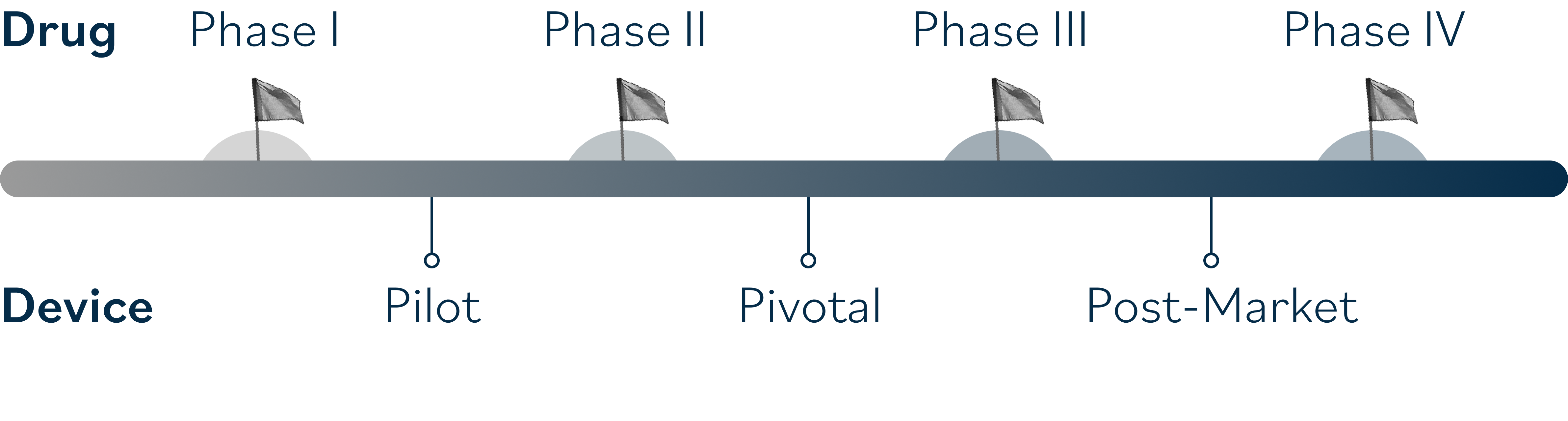
Why Sponsors with therapeutics in the Glaucoma & Optic Neuropathies pipeline choose to partner with Ora
A sample of Ora successes
Expert end-to-end Glaucoma & Optic Neuropathies disease therapeutic pipeline highlights:
Proven track record of success
10+ glaucoma products on the current market were delivered with the help of Ora clinical trials and/or regulatory support.
Strong Partnership
Sponsor-Ora partnership led to the FDA approval of a prostaglandin analogue for the reduction of elevated IOP in patients with open-angle glaucoma, or ocular hypertension.
Successful global Glaucoma trial enrollment
Enrollment of 650+ subjects across 55+ sites in a global Phase 3 Glaucoma clinical trial.
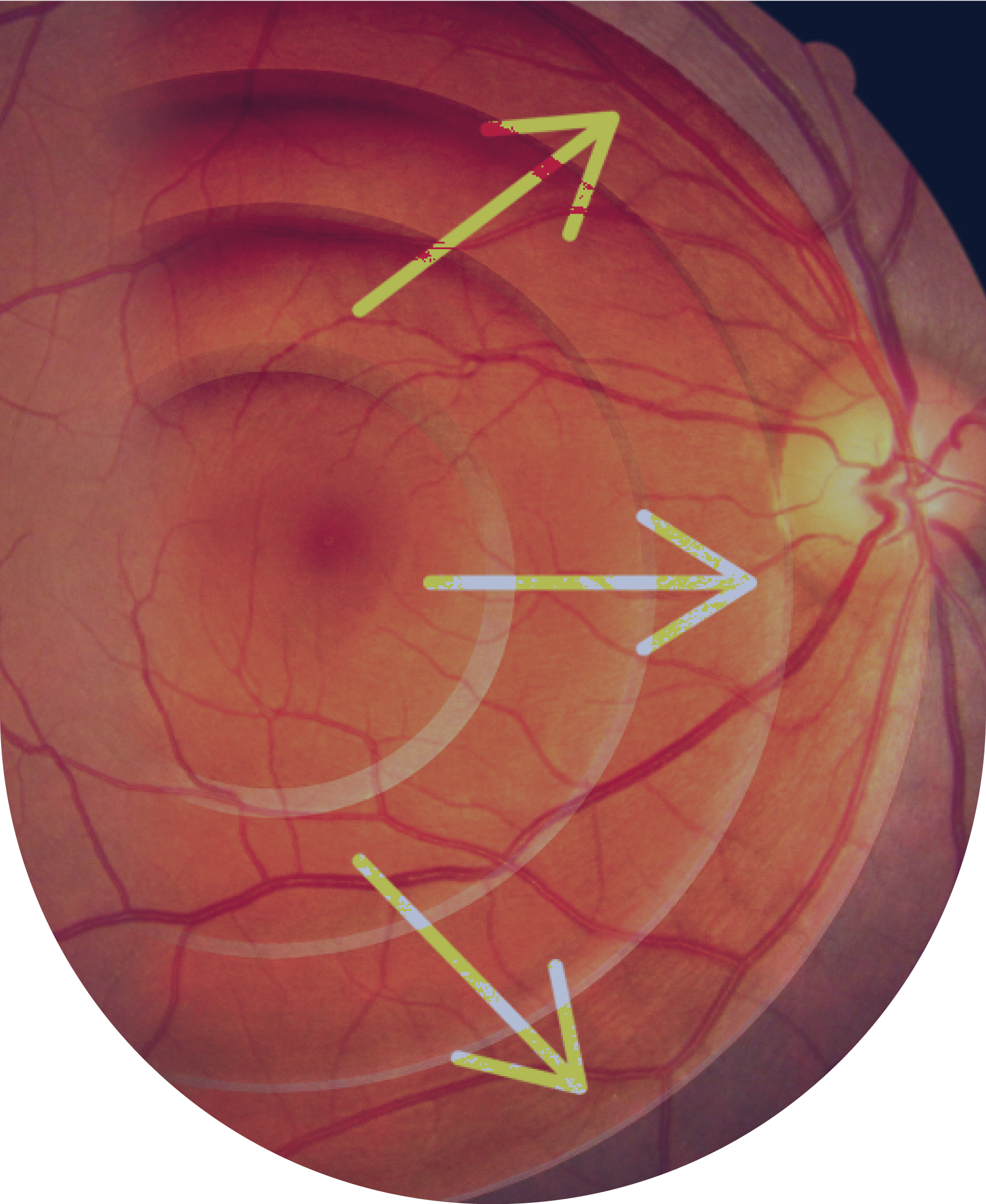
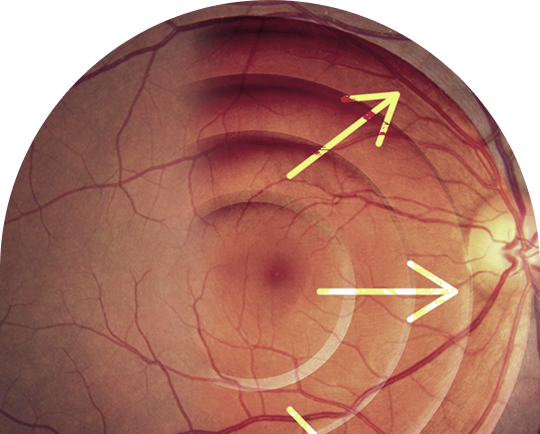
The Ora Difference in Glaucoma Neuroprotection

Over the past five years, Dr. Gus De Moraes has been leading research focused on validating visual field-based endpoints to support regulatory approvals and allow more efficient clinical development timeframes.
Dr. Gus De Moraes and other Ora team members recent (2023) published work in Nature: Scientific Reports, “A potential primary endpoint for clinical trials in glaucoma neuroprotection” has led to us receiving the FDA approval of visual field mean deviation (MD) Slope as a primary endpoint in glaucoma progression clinical trials.
“We harnessed real-world data from over 34,000 glaucoma patients and more than 200,000 visual field tests. Based upon the distribution of disease progression rates in these patients, we identified biomarkers that can accelerate clinical trial timelines. These insights open the door to designing neuroprotection studies with durations of less than two years—bringing therapies to patients faster than ever before,” relayed Dr. Gus De Moraes.
Benefits of the Glaucoma Neuroprotection MD Slope Primary Endpoint
The MD Slope endpoint will allow the implementation of a 2-year or less treatment and clinical trial duration, saving sponsors time and money.
Enrichment of study populations with fast progression (patients with MD slopes steeper than -0.5 dB/year), increasing the likelihood of reaching significant endpoints within shorter durations.
Assessment clustering of visual field comparisons allow the detection of subtle changes in disease progression, allowing for timely adjustments and interventions.
Reduction of study durations and sample sizes without compromising data integrity, accelerating the development of neuroprotective therapeutics.


