Cornea & Ocular Surface
Proven models. An expansive site network. 50+ years of guiding novel therapies to market.
Delivering research for a growing global need.
Cornea & Ocular Surface Diseases—such as dry eye disease (DED), ocular allergy, and neurotrophic and rare surface disorders—are among the leading causes of global vision impairment. Factors like aging populations, increased screen time and resultant eye strain, higher incidences of autoimmune and metabolic disorders, and poor eye care hygiene are driving the demand for more effective, long-term solutions.
The Ora Cornea & Ocular Surface team has 50+ years experience navigating the anterior developmental landscape. In just the past five years, Ora has successfully led 190+ programs—spanning early development through pivotal studies. Our team is uniquely positioned to help sponsors bring breakthrough Cornea & Ocular Surface therapies to market, with deep expertise, proprietary technologies, and a site network built for speed and scale.
Collaborate with Ora, the ophthalmology research company to provide your breakthrough Cornea & Ocular Surface Disease treatment the competitive advantage to win the race to be first to market.
Key Indications
Blepharitis
Dry Eye & Ocular Surface Disease
Ocular Allergy & Inflammation
Ocular Redness
Neurotrophic & Rare Surface Disorders
The Ora Cornea & Ocular Surface team leaders
Ora Cornea & Ocular Surface experience over the last 5 years
Patients enrolled
Sites activated
Programs
The Ora Cornea & Ocular Surface team’s comprehensive expertise spans early-phase clinical trials to late-stage studies. Our strategic regulatory guidance and efficient operational execution, ensures seamless trial progression through even the most complex development pathways.
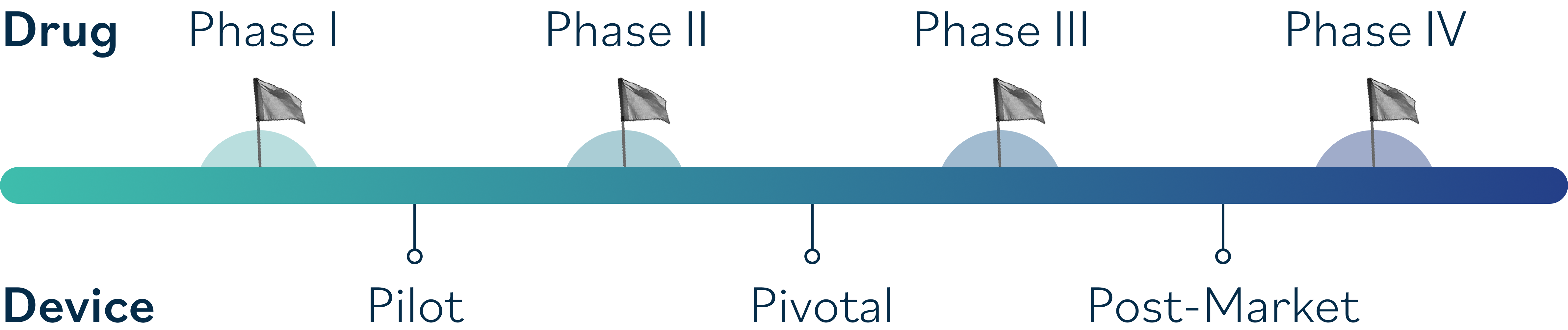
Why Sponsors with therapeutics in the Cornea & Ocular Surface Disease pipeline choose to partner with Ora
A Sample of Ora Successes
Expert end-to-end Cornea & Ocular Disease therapeutic pipeline highlights:
Proven track record of success
With 60+ product approvals for Cornea & Ocular Surface Conditions (Dry Eye Disease, Blepharitis, Ocular Allergy & Inflammation and Ocular Redness).
Proven ability to accelerate enrollment timelines
By utilizing the Ora preferred site network and Ora Block Enrollment, studies can decrease enrollment timelines by 35%.
Provided turnkey support for the Innovative Dry Eye Disease therapeutic VEVYE™
Approved by the FDA in May, 2023 for the treatment of DED.
Ora Cornea & Ocular Surface Endpoints and Technologies
The Ora edge in Cornea & Ocular Surface clinical trials
Ora’s Block Enrollment offering involves concentrating clinical trial visits into a condensed window of time. As a result, sites are able to fully dedicate and prioritize their reseources for clinical research, while making the process of enrollment more efficient. Ora’s unique, protocol-customized approach to screening and enrollment results in a higher volume of patients-per-site. Thus, trials utilizing Block Enrollment require fewer sites, leading to less variability and higher quality data.
To learn more about Ora’s Block Enrollment offering and how it supercharges patient recruitment, please follow the link to the Block Enrollment in Dry Eye & Allergy paper.