With our own Research and Development (R&D) team, Ora believes in our continual evolution to match the best endpoints for each potential therapy.
Historically used endpoints and clinical designs often lack precise control of confounders. As a result, such studies are subject to more “noise” which increases sample size requirements or have low sensitivity to changes within early-stages of the disease. Ora R&D is on a quest for constant improvement of clinical endpoints and approaches to ensure we:

At Ora, we do research on clinical research to optimize and enhance our sponsors’ future clinical trials. When your product has promise, let’s prove it.
For more detailed information please download our one-pager

The Ora Controlled Adverse Environment (CAE®) minimizes influential factors by standardizing temperature, humidity, airflow, lighting, and visual tasking. By creating a constant and reproducible challenge, CAE® offers industry leading precision with fewer patients, fewer sites, and less time.
For more detailed information please download our one-pager

Ora’s IVAD (Inter-blink Interval Visual Acuity Decay) technology provides a clinically relevant, endpoint measurement of visual function. Thus, providing our sponsors with an innovative way to demonstrate therapeutic impact on ocular surface degradation.
For more detailed information please download our one-pager
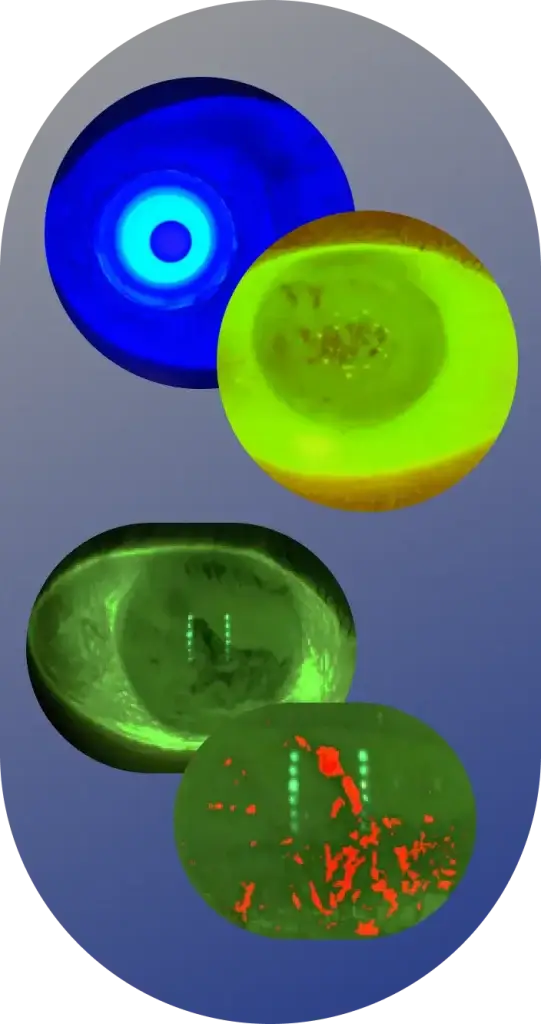
The Ora Ocular Protection Index 2.0 System© provides a precise and novel measurement of the tear film breakup phenomenon and a functional endpoint in a clinical trial.
For more detailed information please download our one-pager

The Ora EyeCup™ technology powered by SDC Capture strengthens the quality of ophthalmic clinical studies, offering a comprehensive solution for capturing patient data and enhancing the overall research process. The user-friendly system captures high-quality clinical data and improves the likelihood of clinical study success.
For more detailed information please download our one-pager
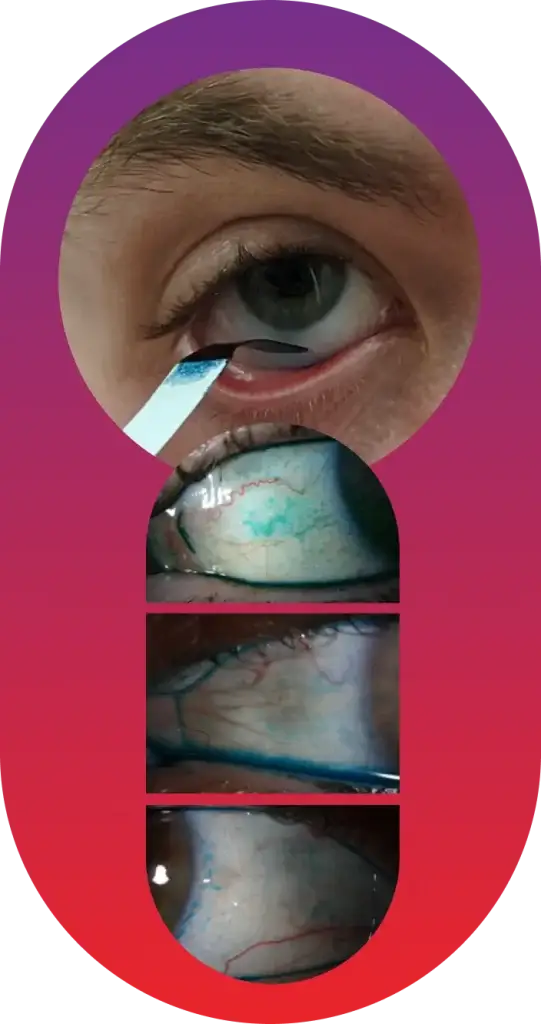
At Ora, the use of Lissamine Green (LG) helps illuminate the entire story of the anterior segment, providing crucial insight into the health and integrity of the conjunctiva.
For more detailed information please download our one-pager
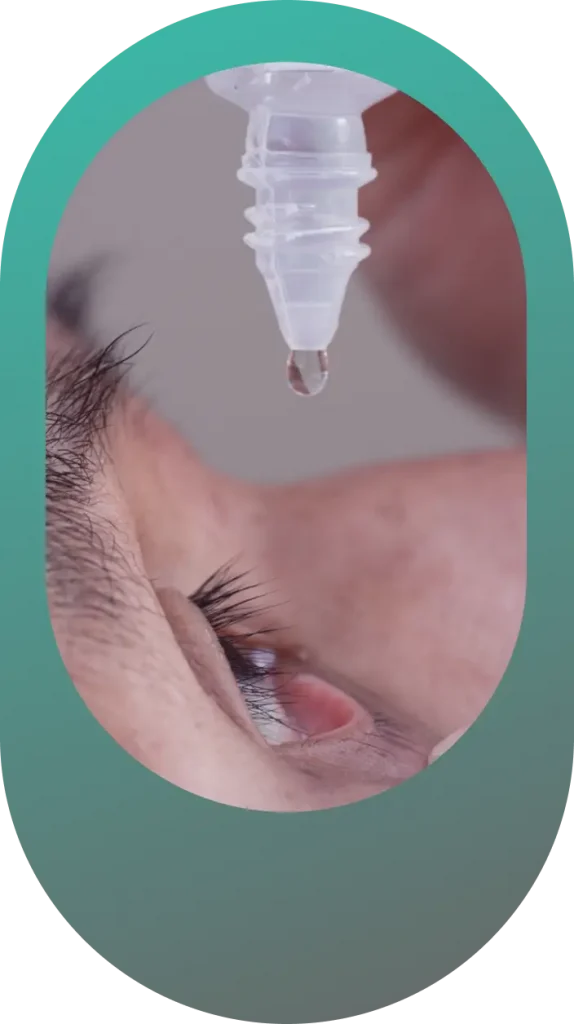
For over 40 years, the Ora conjunctival Allergen Challenge Model (Ora-CAC®) has been used to evaluate every anti-allergic agent for the treatment of allergic conjunctivitis. For the majority of these programs, Ora-CAC® Model studies were pivotal for the FDA approval of the drug.

Ora designed the Visual Navigation Course (VNC™) to assess mobility and functional vision in patients with inherited retinal diseases (IRD). The VNC™ also enables the clinically meaningful assessment of visual function through patient immersion in a 360-degree visual challenge environment.

Ora designed the Visual Navigation Course (VNC™) to assess mobility and functional vision in patients with inherited retinal diseases (IRD). The VNC™ also enables the clinically meaningful assessment of visual function through patient immersion in a 360-degree visual challenge environment.
• The Ora VNC™ is highly adaptable as no single course is appropriate for all IRDs. Some IRDs are characterized by central vision loss (Leber’s Hereditary Optic Neropathy), some by light perception loss (Leber’s Congenital Amaurosis), and others by peripheral vision loss (Retinosa Pigmentosa). We modify the VNC™ to best suit the IRD your therapeutic is designed to treat.
• Ora’s mobility courses have been presented to the FDA as integral endpoints in gene therapy, oligonucleotides, RNA therapy, and medical device development programs. The Ora VNC™ has been accepted by the FDA as a primary endpoint in the United States.
For more detailed information please download our whitepaper.
Immerses patients in a 360-degree visual challenge environment that helps measure therapeutic impact by assessing improvements in patients’ functional mobility.
• Customizable to different difficulty levels to cater to a variety of patient populations and conditions
Clinical research gives you the opportunity to grow your practice and make a difference to those who matter most — patients.