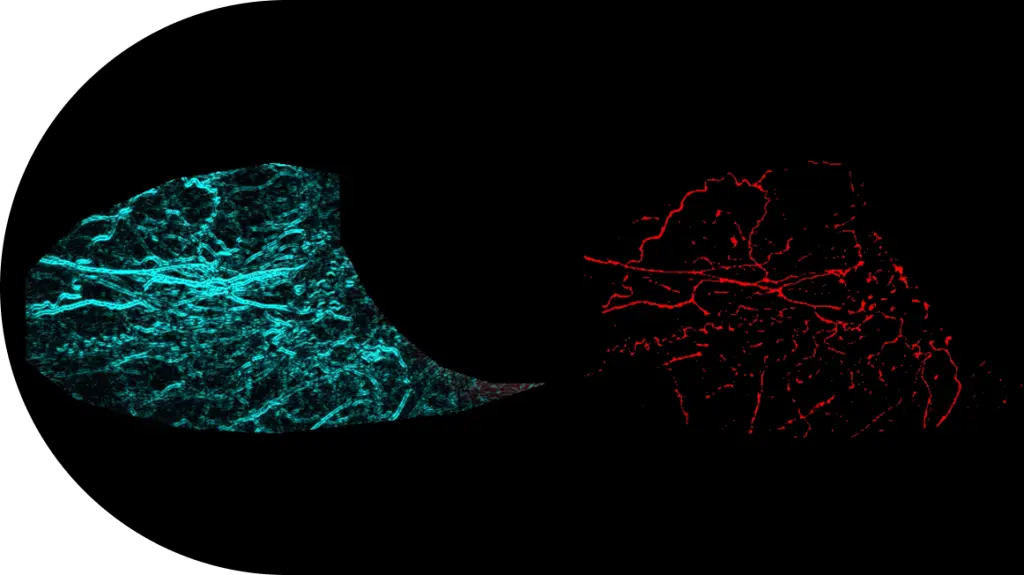
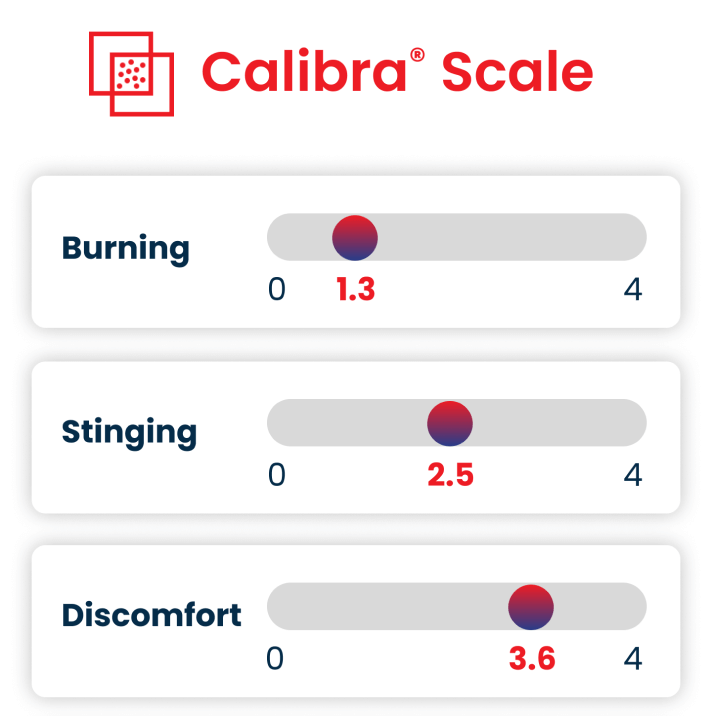
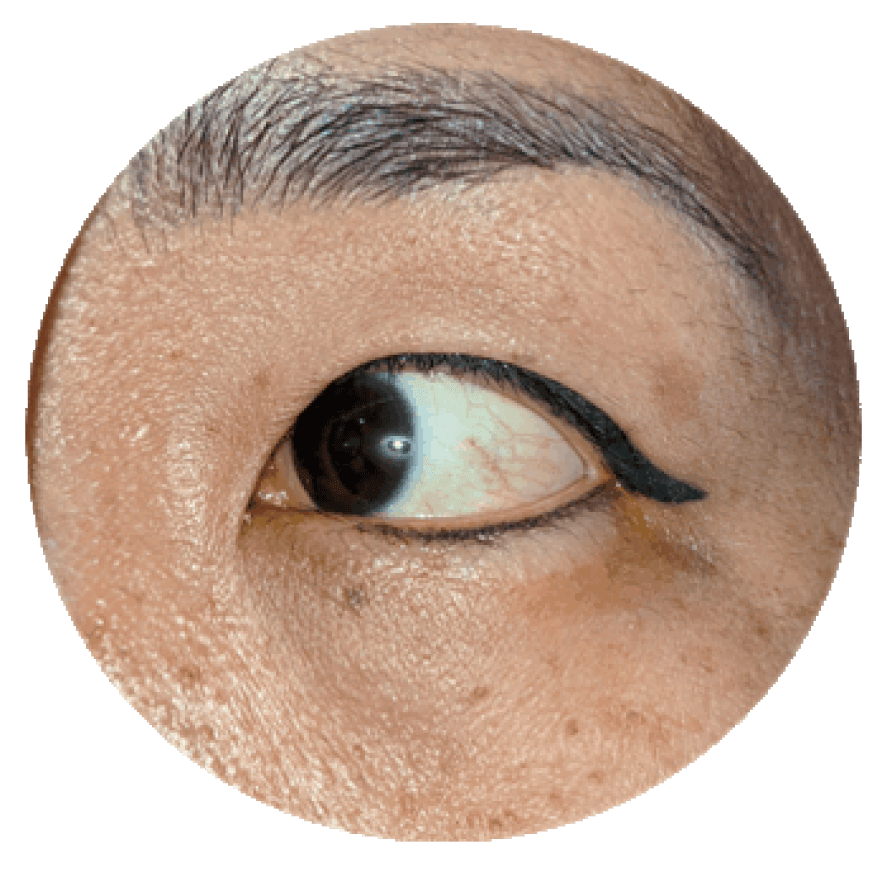
- Expertise With over 3,000 clinical ophthalmology projects completed, our teams are not just experts — they’re THE experts.
- Services We have the right people, processes and technology to streamline research and development and deliver the highest quality results.
- Why Ora As the trusted guide of ophthalmic innovators, we’re here to help navigate the complexities of ophthalmic product development together.
- Company Ophthalmic innovators have trusted our guidance for over 50 years. We’re leading the way in global ophthalmic product development through precision, expertise, and innovation.
- Resources